



Fact:
These products are reviewed by the FDA for quality, safety, and effectiveness.


Myth:
Compounded GLP-1s are just cheaper versions of the same thing
Fact:
They are not FDA-approved, can contain impurities, and may not have the right dose.
As GLP-1 medications like semaglutide and tirzepatide gain popularity, counterfeit and unsafe versions have flooded the market. These underregulated products are frequently promoted through social media ads, influencer campaigns, and online marketplaces, often without requiring a prescription. They may be compounded improperly, mislabeled, or produced in facilities that fail to meet safety and quality standards. Some contain inactive ingredients, incorrect doses, or dangerous contaminants.

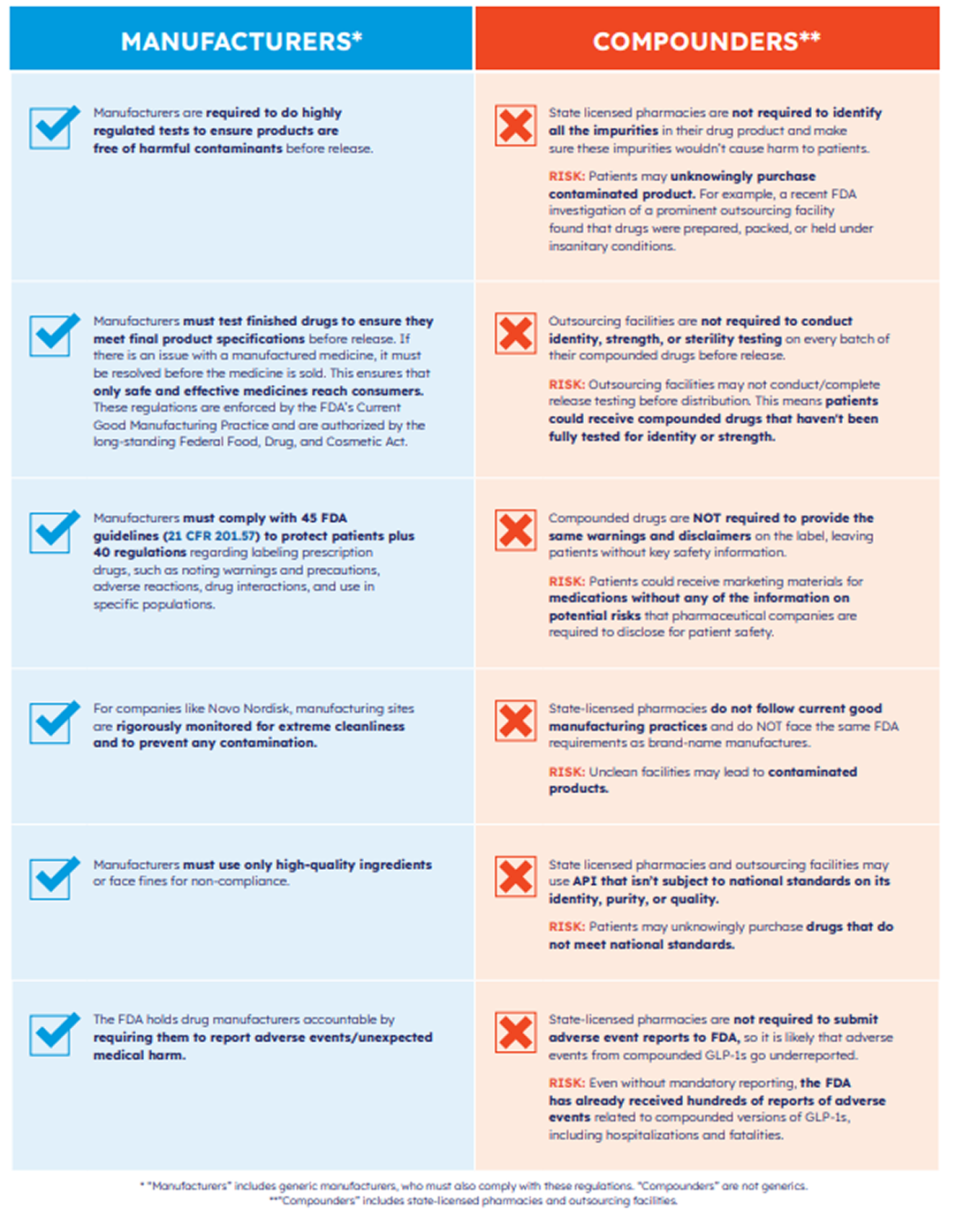
These products are reviewed by the FDA for quality, safety, and effectiveness. Each batch is manufactured under strict standards, with consistent ingredients and dosing. Your prescription is filled by a licensed pharmacy.

Compounding is intended for an individual patient when an approved product is unavailable or a patient needs a customized form (e.g., allergy, different dosage form). Compounded versions are not FDA‑approved and may vary in strength, purity, and stability.
Fake products can contain the wrong ingredient or the wrong amount of drug, be contaminated by poor manufacturing practices, and come with vague or misleading labels that make safe dosing impossible. Multi‑use vials that require you to self‑measure doses are especially risky and have led to overdoses and hospitalizations. If anything about the product, packaging, or seller feels off, do not inject it—contact a licensed pharmacist or your prescriber right away and report the incident to FDA MedWatch.
Fake products can contain the wrong ingredient or the wrong amount of drug, be contaminated by poor manufacturing practices, and come with vague or misleading labels that make safe dosing impossible. Multi‑use vials that require you to self‑measure doses are especially risky and have led to overdoses and hospitalizations. If anything about the product, packaging, or seller feels off, do not inject it—contact a licensed pharmacist or your prescriber right away and report the incident to FDA MedWatch
Fake products can contain the wrong ingredient or the wrong amount of drug, be contaminated by poor manufacturing practices, and come with vague or misleading labels that make safe dosing impossible. Multi‑use vials that require you to self‑measure doses are especially risky and have led to overdoses and hospitalizations. If anything about the product, packaging, or seller feels off, do not inject it—contact a licensed pharmacist or your prescriber right away and report the incident to FDA MedWatch
Fake products can contain the wrong ingredient or the wrong amount of drug, be contaminated by poor manufacturing practices, and come with vague or misleading labels that make safe dosing impossible. Multi‑use vials that require you to self‑measure doses are especially risky and have led to overdoses and hospitalizations. If anything about the product, packaging, or seller feels off, do not inject it—contact a licensed pharmacist or your prescriber right away and report the incident to FDA MedWatch
Compounded GLP‑1s may only be prepared under narrow conditions (e.g., a specific medical need not met by an approved product).
Bulk API from unverified foreign suppliers can be detained at the border; products labeled “research use only/not for human use” are not appropriate for patients.

Using salt forms (e.g., semaglutide sodium/acetate) is not equivalent to approved drugs and should not be used for compounding.